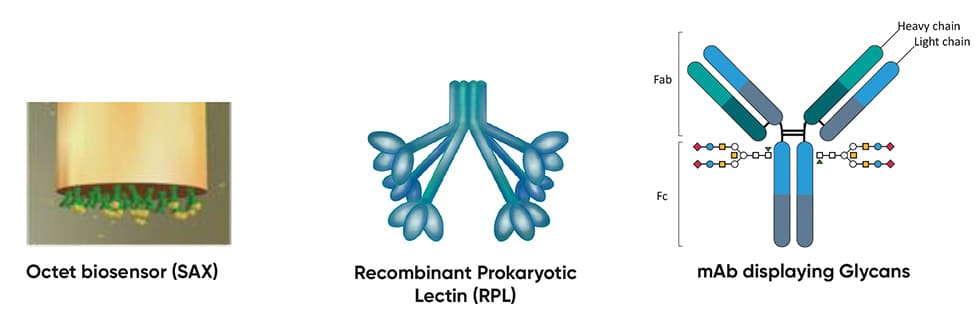
Development of Simple, Rapid & High-Throughput Glycoanalytics for Biopharmaceuticals
Introduction
Glycosylation is a post-translational modification that can influence the safety and efficacy of biologic drugs, making it a Critical Quality Attribute for biotherapeutic manufacturing. It is typically characterised using lectins (bioaffinity proteins that recognize and bind specific glycan structures) for HPLC and MS-based analysis. However, these methods have limited throughput and rely on plant-based lectins that lack specificity and are difficult to produce recombinantly. GlycoSeLect’s Recombinant Prokaryotic Lectins (RPLs) offer superior specificity, consistency, and scalability over plant-based lectins to streamline the analysis of intact glycosylated biomolecules. By integrating RPLs into the Sartorius Octet system for glycoanalysis of biotherapeutics provided by Allergan Biologics, and assay validation by CPI, this project aimed to develop a novel platform for simpler, faster glycoprofiling.
Method
Two biopharmaceutical samples, follicle stimulating hormone (FSH) and the Fc fusion protein Eylea, were selected for glycoanalysis using the Sartorius Octet, a label-free detection system based on biolayer interferometry. Briefly, RPL-Gal1 and RPL-Sia1 were immobilised on biosensors via the biotin-streptavidin interaction and used to monitor terminal β1-4 galactose and sialic acid structures, respectively. Assay robustness was assessed by determining the linearity, repeatability, and percentage spike recovery of each assay.
Method Overview
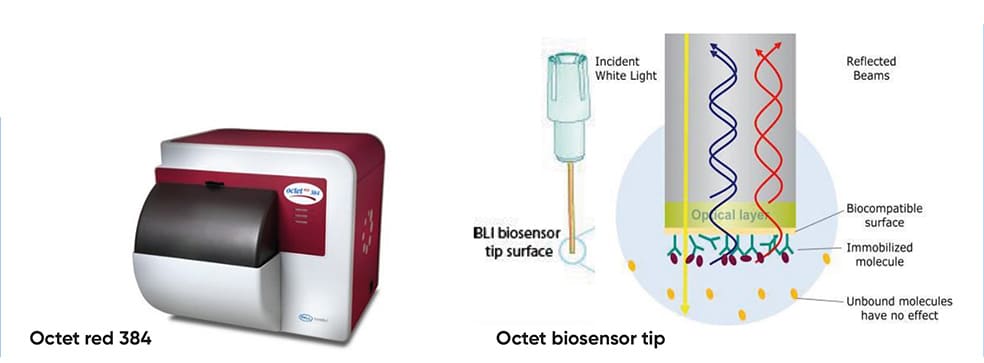
(A) The Octet biosensor tip Figure 1 (A) – Octet red 384; (B) – Octet red 384 stage; (C) – over time graph; (F)Binding rate over concentration
(B) RPL immobilisation protocol
|
Step |
Buffer |
Duration |
Agitation |
|
Baseline |
TBST |
60sec |
1000rpm |
|
Loading |
RPLs (at20μ/ml) |
600sec |
1000rpm |
|
Wash |
TBST |
180sec |
1000rpm |
(C) Concentration ranges evaluated for each RPL-Biopharmaceutical pair
|
RPL- Biopharmaceutical pair |
Linearity range (μ/ml) |
Spike recovery Concentration (μ/ml) |
Repeatability Concentration (μ/ml) |
|
Gal1-FSH |
500-7.8 |
50, 25, 12.5 |
200 |
|
Gal1-Eylea |
500-15.6(≥15.6) |
N/A |
200 |
|
Sia1-FSH |
150-1.2 |
15, 7.5, 1.9 |
50 |
|
Sia1-Eylea |
250-7.8 |
N/A |
100 |
Results
Linearity
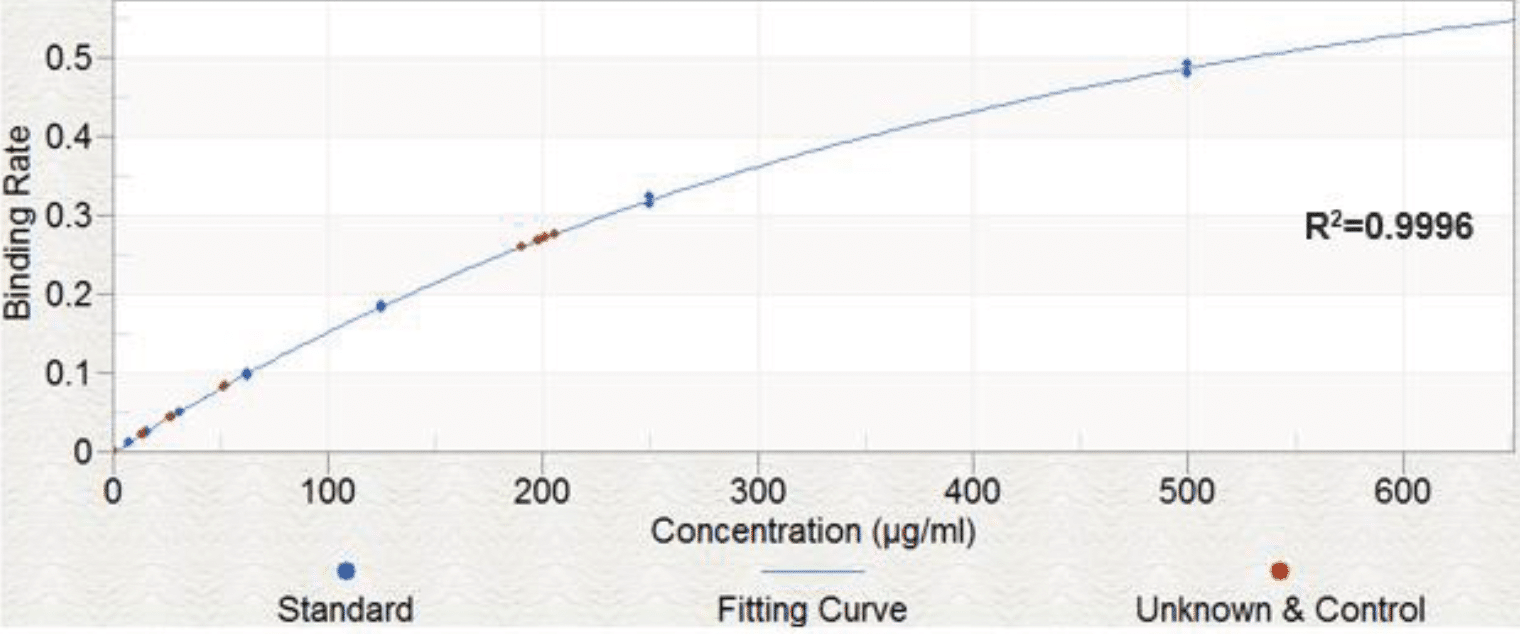
The linearity of FSH (β1-4 galactose) analysis was determined by measuring the binding rate of two replicates (n=2) at 8 concentrations and demonstrated an acceptable range for the assay of 7.8 to 500 μg/ml (%CV <20%).
Data is shown in Figure 1
A
|
FSH (μg/ml) |
Conc. Avg. (μ/ml) |
%CV |
|
500.0 |
500.2 |
3.65 |
|
250.0 |
250.0 |
2.55 |
|
125.0 |
125.1 |
2.44 |
|
62.5 |
62.4 |
3.00 |
|
31.3 |
31.3 |
0.53 |
|
15.6 |
15.7 |
1.71 |
|
7.8 |
7.8 |
1.07 |
|
0 |
0.0 |
0.00 |
B
Repeatability
Assay repeatability and precision was assessed by measuring six replicates of FSH at 200 μg/ ml. Data are shown in Figure 1, with concentration results in Figure 2. The assay showed good repeatability with a %CV of <10% for FSH quantitation.
|
Octet analysis of FSH (β1-4 galactose) |
|
|
Repetition |
FSH at 200 μg/ml |
|
1 |
190.4 |
|
2 |
197.7 |
|
3 |
198.4 |
|
4 |
200.9 |
|
5 |
205.8 |
|
6 |
197.6 |
|
Conc. Avg. |
198.5 |
|
%CV |
2.53 |
Figure 2. FSH (β1-4 galactose) concentration results.
Spike Recovery
Percentage spike recovery tests to investigate matrix interference during quantitation of FSH (β1-4 galactose) showed recoveries of 80-120% with a %CV of <10%, indicating that glycan detection is not compromised by the presence of the sample buffer (Figure 3).
|
Known Conc. (μg/ml) |
Well 1 Conc. (μg/ml) |
Well 2 Conc. (μg/ml) |
Avg. Conc. (μg/ml) |
%CV |
% Spike Recovery |
|
12.5 |
13.8 |
14.1 |
14.0 |
1.52 |
112% |
|
25.0 |
27.5 |
27.0 |
27.3 |
1.30 |
109% |
|
50.0 |
52.1 |
52.0 |
52.1 |
0.14 |
104% |
Figure 3. Percentage spike recovery for FSH (β1-4 galactose) from a matrix comprising a mix of FSH formulation buffer and assay buffer.
Similar spike recoveries were seen for FSH (sialic acid), and for both Eylea assays, which all demonstrated high intra-assay linearity and repeatability. Figure 6 shows the standard curves obtained for these samples.
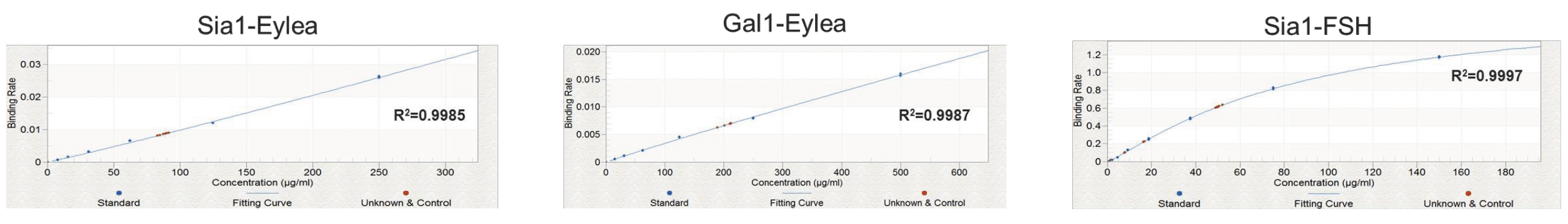
Conclusion/Summary
The integration of RPLs into the Sartorius Octet system represents a novel, high-throughput method for detecting glycans in purified biopharmaceutical samples. Assay robustness is validated by high-linearity, repeatability, and percentage spike recovery, and confirms the viability of this approach for simpler, faster glycoprofiling.
Acknowlegments
This project consortium brought together the expertise and resources of CPI, GlycoSeLect UK Ltd, ForteBio Pall Life Science (now part of Sartorius Group) and Allergan Biologics Ltd to address the need for new glycoanalytical methods within the growing biotherapeutics market.